Recently, the research team led by Chen Yuanzheng at the School of Physical Science and Technology, Southwest Jiaotong University, has made significant advancements in the field of electrocatalysis. Their research, titled "The activity origin of C-N-Cu electrocatalysts for ethanol formation in the CO2 reduction reaction under working conditions," was published in the prestigious core journal "J. Mater. Chem. A" (IF=11.9) under the Royal Society of Chemistry. Zhang Xiaotao, a master's student at the school, served as the first author of the paper, with Associate Professor Chen Yuanzheng acting as the supervising teacher and corresponding author.
The precise identification of catalytic active sites and the effective tracking of product information are crucial for elucidating the micro-mechanisms of electrocatalytic reactions. This forms the basis for establishing a strict structure-activity relationship to guide the design of efficient catalysts. However, with the continuous development of in-situ characterization technologies in recent years, it has been confirmed that many catalysts undergo various degrees of restructuring under reaction conditions, hindering a precise understanding of the active centers and catalytic mechanisms in these reactions. Therefore, revealing the true active centers of catalysts under working conditions and their catalytic mechanisms is of significant importance.
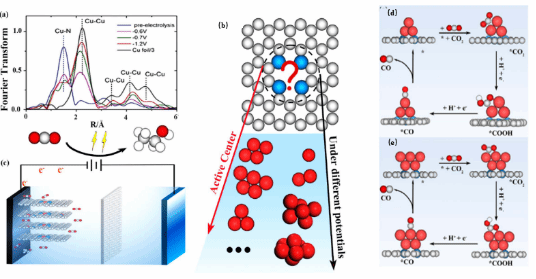
The copper-anchored nitrogen-doped graphene (C-N-Cu) single-atom catalyst has been experimentally proven to exhibit high catalytic activity in the electrochemical reduction reaction of CO2 to ethanol. Existing studies suggest that the high activity of C-N-Cu catalysts mainly originates from the formation of Cu clusters by Cu atoms under working conditions. However, current in-situ techniques are unable to effectively characterize the transient Cu cluster structures formed during the reaction process, presenting a significant challenge in exploring the catalytic activity centers. This work employed a constant potential simulation method, considering both electrode potential and solvation effects, to theoretically reveal the active centers of C-N-Cu catalysts under working conditions and provide a complete reaction mechanism on them. The research indicates that when a negative potential is applied to the C-N-Cu catalyst, electrons tend to occupy the antibonding orbitals of the Cu-N bond, driving the precipitation of Cu atoms to form Cu clusters. By simulating the reaction Gibbs free energy of different products on different single-atom cluster catalyst configurations with voltage changes and comparing the experimentally obtained product distribution trends, the study identified the C-N-Cu5 structure as the true active center under reaction conditions. The d-band center, charge distribution, and multiple active sites of C-N-Cu5 ensure it to be an ideal platform for the reduction of CO2 to ethanol. Based on this active center, the study further elucidated the reaction mechanism of ethanol production on the C-N-Cu5 catalyst and key intermediates, revealing the adsorbed CHO species as a crucial reactant in the C-C coupling reaction. These findings provide a comprehensive understanding of the catalytic activity centers and reaction mechanisms of C-N-Cu catalysts under working conditions, offering insights into the dynamic behavior of catalysts at a microscopic level.
This work was supported by the National Natural Science Foundation of China (12164009), the Science and Technology Department of Sichuan Province (2022ZYD0024, 2021YFG0228), the Natural Science Foundation of Hebei Province (E2022203109), and the China Postdoctoral Science Foundation (2021M690325).
Paper Information: J. Mater. Chem. A, 2024, 12, 3580–3588
Paper Link: https://pubs.rsc.org/en/content/articlelanding/2024/ta/d3ta05325c





